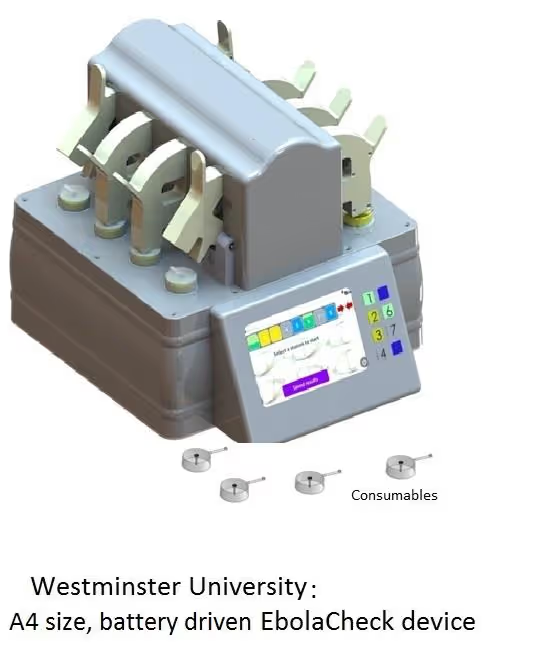
EbolaCheck

Project overview
Ebola-check is a point-of-need diagnostic device suitable for simple, rapid and safe patient triage at treatment centres anywhere in West Africa.
Countries
Guinea
Organisations
University of Westminster
Partners
BioGene Ltd, US Army Medical Research Institute of Infectious Diseases, Kwame Nkrumah University of Science and Technology, Public Health England
Area of funding
Humanitarian Research
Grant amount
£629,655
Start date
01
November
2014
End date
01
November
2015
Project length (in months)
12
Project solution
This project offers [specific solution or intervention] to tackle [challenge]. By implementing [strategies, tools, or innovations], the project aims to achieve [desired outcomes]. The approach is designed to [specific actions or methods] to bring about meaningful change in [community, region, or issue area].
Expected outcomes
This project aims to achieve [specific outcomes], such as [measurable results, improvements, or changes]. The expected impact includes [benefits to the target community, advancements in research or innovation, or long-term effects]. By the end of the project, we anticipate [specific changes or milestones] that will contribute to [broader goals or objectives].
Principal Investigator: Sterghios A. Moschos, University of Northumbria
Purpose
Symptomatic patients arriving at Ebola treatment centres in West Africa were triaged by a combination of reviewing their symptoms, assessing Ebola epidemiology in their community, and confirming infection through molecular diagnosis. Beyond acute high fever, symptoms that raise suspicion of Ebola Virus Disease (EVD) include vomiting, diarrhoea and uncontrolled haemorrhage. Importantly, these coincide with high levels of viremia and contagiousness through contact with bodily fluids, and are also symptomatic of other diseases prevalent in West Africa, such as malaria.
It is thus necessary to quickly identify and isolate EVD patients to prevent further transmission. However, until that crucial negative molecular result is returned, patients that might be Ebola-free are cared for side-by-side with confirmed positive cases, at risk of acquiring the disease and death. Minimising the time to molecular diagnosis would therefore greatly improve the chances of controlling Ebola outbreaks.
;
Outcomes
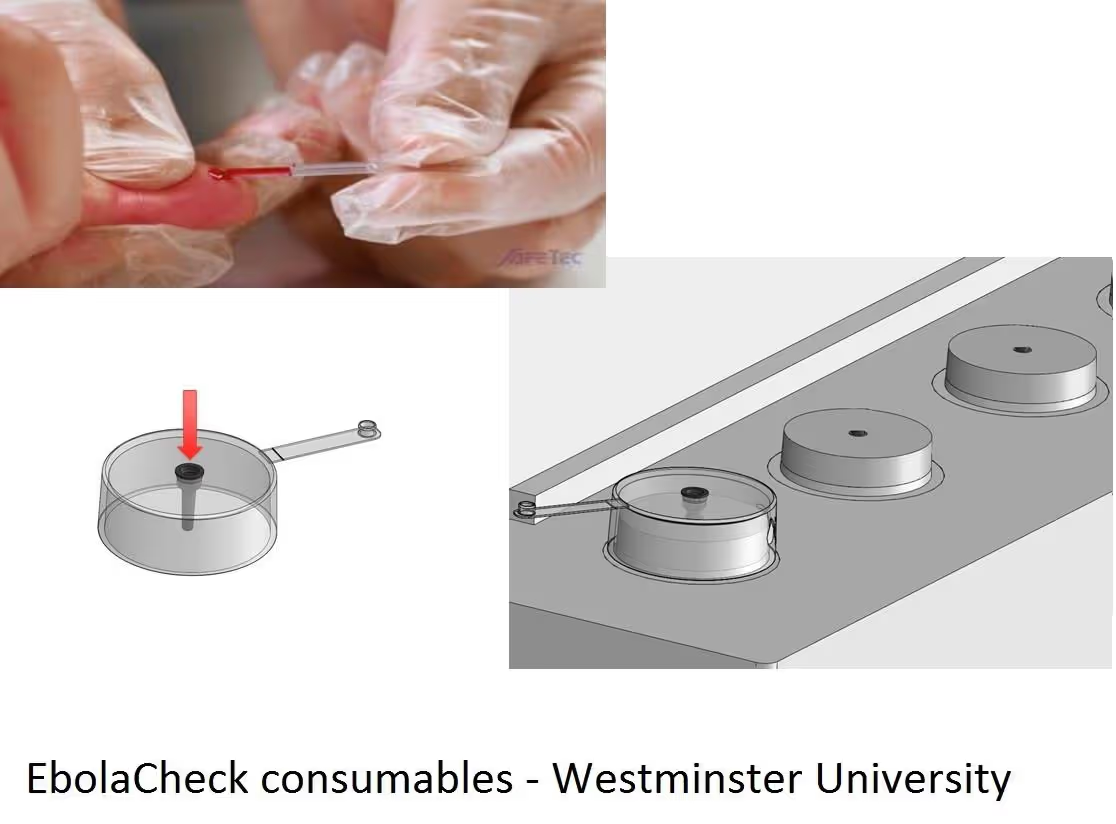
The project has delivered ‘EbolaCheck’, a point-of-need diagnostic device suitable for simple, rapid and safe patient triage at treatment centres anywhere in West Africa. The approach is based on a combination of a validated, proprietary technology for rapid, direct polymerase chain reaction (PCR) on whole blood samples [ExSyte (EBVK8), based on WO2011157989] and the standard of care RTKPCR laboratory diagnostic assays used worldwide to confirm Ebola infection. The development of this diagnostic device has helped enhance patient triage and improve healthcare worker safety.
Key Findings
- One of the key findings was that reverse transcription, the process of converting RNA molecules such as the Ebola genome into DNA, is possible in crude blood, serum and cerebrospinal fluid samples. The study also demonstrated that quantification of genetic material in such crude samples is possible to the extent that the biomarker being assessed is found in concentrations ranging from a few copies to a few billion copies. The capacity to detect <;10 copies of virus up to 6 billion, whether the virus has or does not have a lipid envelope, suggests that a wide variety of viruses can be reliably detected and quantified using this technology.
- The solution achieved through EbolaCheck can reliably discriminate between log-range differences in viraemia as simulated by spiking mock patient sample (fresh blood and serum) with surrogate viruses or ebolavirus obtained from cell culture preps. Thus, whilst many alternatives were put forward during the Ebola outbreak, from pregnancy test-styled dip sticks to table top lab automation systems or manually and technically demanding ‘lab in a suitcase’ approaches, few combine the laboratory-free portability, simplicity and quantifiable data nature of EbolaCheck. The level of discrimination offered on viraemia, coupled to the data on mortality risk suggests future utility in reliably predicting the risk to the patient on arrival at triage or during treatment.
Key outputs
- 1 peer reviewed article published (see ‘Publications’ section below for details)
- Single well, triplex testing instrumentation that is compatible at least with crude blood and cerebrospinal fluid at a final, pre-scale up cost per patient of US$12.
- Capability to quantify, not just detect Ebolavirus.
- ‘Gold standard’ assay migration onto EbolaCheck to achieve better performance than published assay capability.
- Migration of one more Ebola and one control assay in addition to the minimal assay in EZ1.
- Proof of principle that the platform may discriminate vaccinated from infected individuals and confirm system operational reliably.
- Validation of ‘gold standard’ reagent mix utility on the instrument (refrigerated consumables) with live Ebola.
- Evidence of utility for capsid viruses, lipid envelop viruses, gram positive and gram negative bacteria.
- Cold chain free reagent mix development compatible with EbolaCheck technology and with minimal impact on quantitative capability has been developed but not validated with live Ebola.
- Demonstration of rapid (<;3 weeks) transfer of existing clinical assays onto the EbolaCheck platform to meet emerging need.
- Instrument fully compatible with chemical sterilisation and rapid redeployment-reuse.
Next steps
- In January 2018, it is planned to field test EbolaCheck in rural areas in Brazil with over 400 patients to test potential challenges like humidity and temperature, and the simplicity of the operating procedure (https://www.scidev.net/global/ebola/feature/ebola-pinprick-field-trial.html)
[.slimline-cta-box][.slimline-cta_heading]R2HC Funding for Ebola Projects. A Rapid Response[.slimline-cta_heading][.slimline-cta_paragraph]In August 2014, the Ebola Outbreak in West Africa was declared an International Health Emergency by WHO and within a couple of weeks ELRHA launched a rapid-response call for research to combat the crisis. The UK Department for International Development (DFID), the Wellcome Trust and ELRHA opened a special funding window through the Research for Health in Humanitarian Crises (R2HC) programme.
The aim of this emergency call was both to produce robust research findings that could contribute to the effectiveness of the response to the current outbreak and help to draw lessons for future outbreaks of Ebola and other communicable diseases. The projects funded will strengthen the evidence base for the Ebola response in topics ranging from diagnostics to anthropology, surveillance and disease control.[.slimline-cta_paragraph][.slimline-cta-box]
No items found.
Project delivery & updates
Stay up to date with the latest developments from this project. Here, you will find details on what has been delivered, resources created, and regular updates as the project progresses. Access key documents, reports, and other materials to see how the project is making an impact.
No resources/updates have been published yet for this project. Sign up for our newsletter to stay informed about upcoming publications and updates!
Join our Newsletter
Resources
Field-deployable, Quantitative, Rapid Identification of Active Ebola Virus Infection in Unprocessed Blood.
Journal article
LEARN MORELatest updates
No items found.