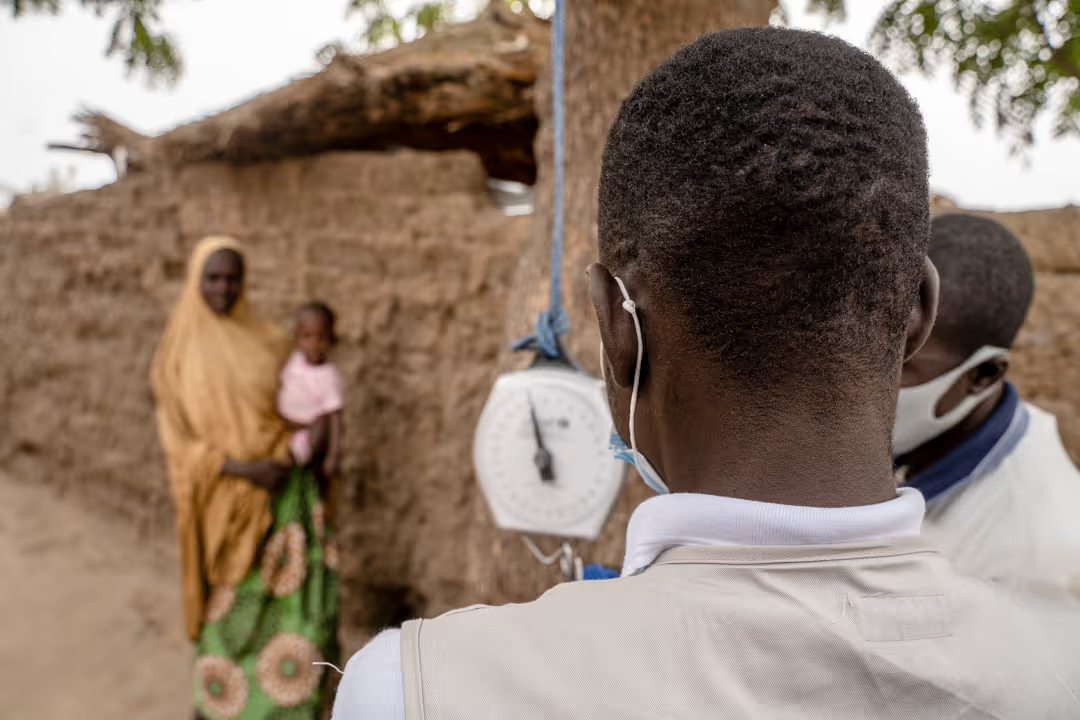Simplified and optimised management of acute malnutrition in children aged 6 to 59 months: OptiMA-Niger, a community-based clinical randomized controlled trial in the Mirriah district in Niger

Project overview
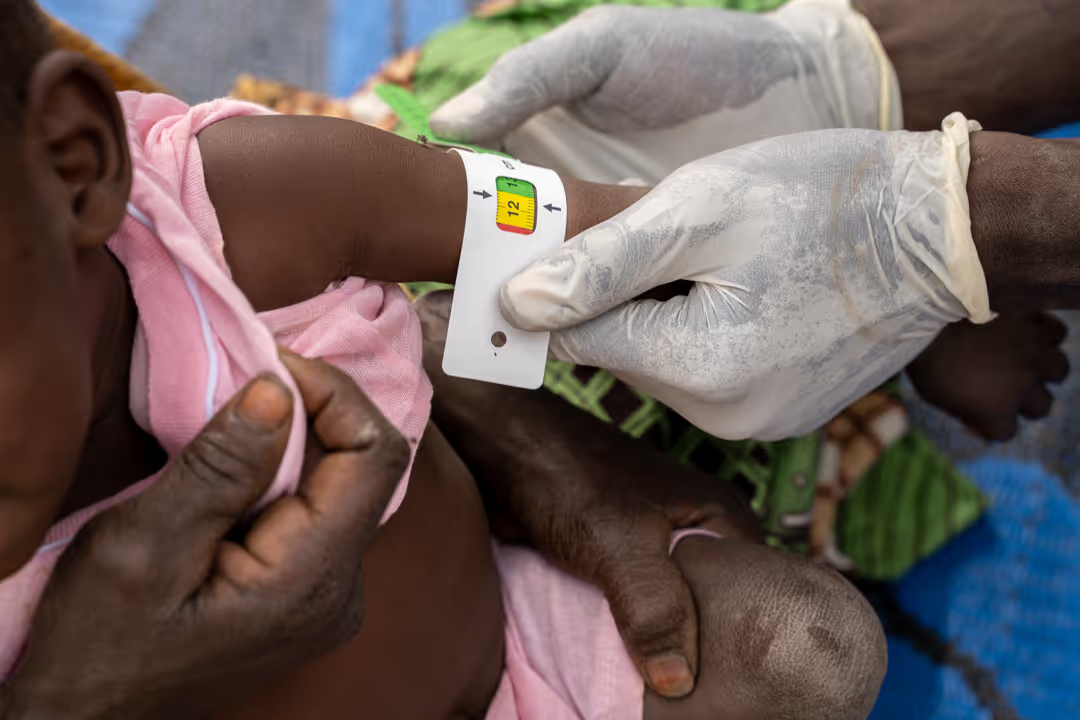
This study is investigating the effectiveness of a MUAC-based protocol for the treatment of severe and moderate acute malnutrition in a single protocol.
Countries
Niger
Organisations
Alliance for International Medical Action (ALIMA)
Partners
INSERM, France, Haut Commissariat a L’Initiative les Nigeriens Nourissent les Nigeriens (HC3N), Niger, Bien Etre de la Femme et de l’Enfant au Niger, (BEFEN).
Area of funding
Humanitarian Research
Grant amount
£606,424
Start date
01
September
2019
End date
01
February
2023
Project length (in months)
41
Funding calls
Focus areas
Topics
Nutrition
Status
Live
Project solution
This project offers [specific solution or intervention] to tackle [challenge]. By implementing [strategies, tools, or innovations], the project aims to achieve [desired outcomes]. The approach is designed to [specific actions or methods] to bring about meaningful change in [community, region, or issue area].
Expected outcomes
This project aims to achieve [specific outcomes], such as [measurable results, improvements, or changes]. The expected impact includes [benefits to the target community, advancements in research or innovation, or long-term effects]. By the end of the project, we anticipate [specific changes or milestones] that will contribute to [broader goals or objectives].
Principal Investigator: Susan Shepherd, ALIMA & Renaud Becquet, INSERM
Purpose
The study in Niger aims to investigate the effectiveness of a mid-upper arm circumference (MUAC)-based protocol for the treatment of severe and moderate acute malnutrition in a single protocol in young children, compared to the standard protocol. The study will address a significant evidence gap about the treatment of acute malnutrition, and how this can be carried out in a more effective manner that enables children to be treated earlier and more children to be reached.
Expected Outcomes
Findings will be highly applicable to other settings, especially in Francophone West Africa where there are multiple countries which have recurrent malnutrition crises. The study will make an important contribution to the global evidence base on the effectiveness of protocols which integrate severe and moderate acute malnutrition treatment, where multiple studies are likely to be needed to counter the potential institutional opposition to diverging from existing protocols.
Research Methodology (summary)
In this individually randomized nutrition trial, all children 6-59m in selected villages will be screened monthly to identify those who meet inclusion criteria. These children will be referred to the health centre for consent and inclusion, where each study participant will either be prescribed Ready-to-use Therapeutic Food (RUTF) (if eligible according to his/her randomisation arm), or monitored bi-monthly for health and nutrition status (if not RUTF-eligible) for a period of 6 months.
No items found.
Project delivery & updates
Stay up to date with the latest developments from this project. Here, you will find details on what has been delivered, resources created, and regular updates as the project progresses. Access key documents, reports, and other materials to see how the project is making an impact.
No resources/updates have been published yet for this project. Sign up for our newsletter to stay informed about upcoming publications and updates!
Join our Newsletter
Resources
No items found.
Latest updates
No items found.